
The World Health Organization (WHO) is currently conducting an online Public Hearing to collect data and proposals from various stakeholders and actors, towards an analysis of intellectual property rights, innovation and public health. Running 1-15 November, the Public Hearing will accept contributions from a wide range of stakeholders and actors.
The contributions to the Public Hearing will be collected for the first official meeting (4-8 December) of the intergovernmental working group (IGWG), convened under Resolution WHA59.24, "Public health, innovation, essential health research and intellectual property rights: towards a global strategy and plan of action." Resolution WHA59.24 was adopted at the 59th World Health Assembly, after consideration of the recommendations of the WHO Commission on Intellectual Property Rights, Innovation and Public Health (CIPIH), which concluded with its report in April 2006.
The IGWG is open to all interested Member States, and IP-Watch reports that the WHO will also invite a wide range of observers, UN organisations, intergovernmental and nongovernmental organisations, as well as experts and selected public and private entities.
The IGWG will collect the data and proposals from various stakeholders through this Public Hearing and prepare an analysis of intellectual property rights, innovation and public health, to be submitted to the 61st World Health Assembly, to be held in May 2008. This analysis will develop a global strategy for sustainable needs-driven health research and development, with particular attention to those diseases affecting developing countries.
Some of these major diseases are those caused by helminth infections. Earlier this month, the WHO published guidelines on Preventive Chemotherapy in Human Helminthiasis, examining large-scale prevention and treatment programmes in helminth infections causing schistosomiasis or bilharzia, diseases which are disproportionately high in developing countries. The manual sets out the new strategy to fight these infections, involving a partnership of more than 25 organisations. As well as a significant public health concern, the WHO has also identified serious considerations with respect to human rights, in that sufferers are often ostracised and stigmatised as a result of these infections and the effects on the body. In the accompanying press release to the publication, Dr David Heymann, WHO Acting Assistant Director-General for Communicable Diseases, said that treatment and prevention of these diseases is urgent and "incontestable from all perspectives: moral, human rights, economic and global public good. The task is feasible and must be done."
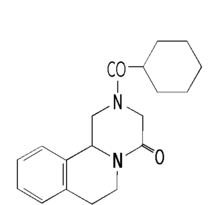
Pharmaceutical companies and private donors "make good the gap" in delivering the support to poorer countries, as reported in the Financial Times (FT) earlier this month. According to the FT, all the drugs required for these are diseases are donated, except Praziquantel (for the treatment of bilharzia, formula at right). Merck manufactures the drug, and Bayer markets the drug for veterinary use. WHO has been in negotiations with both for some time, but to date there had been no agreement to provide the drug.
However, Merck is now co-operating with the WHO towards the production by 2008 of drugs to treat bilharzia at less than half the market cost, following reports of increased "pressure" on Bayer and Merck to donate "for human use a drug they sell for pets." Professor Lorenzo Savioli, Director of the WHO Department of Control of Neglected Tropical Diseases, said to the FT that the renewed action and fresh requests to the companies are in response to "a real market failure because schistosomiasis affects the poorest of the poor. But Bayer tells us to talk to Merck, and Merck says talk to Bayer."
The FT reports that Merck was approached by the WHO early this year in Spring, but that supplies were presently too small and production costs too high to allow for a viable mechanism to supply the drug at the costs and quantities required.
The FT also reports that Bayer originally helped develop praziquantel with WHO support in the 1970s for the treatment of patients in Africa. However, Bayer concluded that it could not produce the drug so that it would be affordable. Bayer's public profile, much maligned following the GM contamination of US rice imports, is unlikely to be assisted by what the FT reports as its reluctance to participate in initiatives to achieve distribution of the drug in Africa.
Meanwhile, Pfizer is providing open access to its library of 3 million chemical compounds to WHO-affiliated researchers on tropical diseases. Initially, access was granted to 12 000 compounds for the treatment of helminth infections and related concerns. The intellectual property rights arising from such programmes, however, are not clear, as no decision has been made on what will happen to any promising developments.

No comments:
Post a Comment